Ceramic materials are brittle hard strong in compression and weak in shearing and tension.
Key chemical properties of ceramics.
A ceramic material is an inorganic non metallic often crystalline oxide nitride or carbide material.
Chemical durability against the deteriorating effects of oxygen water acids bases salts and organic solvents.
Different materials have different properties.
Different materials have different properties.
Low to medium tensile strength.
Advanced ceramics fine ceramics possess good chemical stability.
Great hardness and strength.
Polymers are strong and tough and often flexible.
Polymers are strong and tough and often flexible.
Basically these bonds result in good chemical resistance but have the low thermal expansion high melting point and hardness.
Mass properties e g density ceramics are intermediate density 2 00 6 00 gms cm3 different for allotropes e g glass cristobalite tridymite quartz 2.
Mechanical strength in spite of brittleness.
Chemical inertness they re unreactive with other chemicals.
Ceramics exhibit very strong ionic and or covalent bonding stronger than the metallic bond and this confers the properties commonly associated with ceramics.
Thermal and electrical conductivity considerably lower than that of metals.
Low to medium thermal conductivity.
Low electrical and thermal conductivity they re good insulators.
Composite materials combine two or more materials.
Ceramics are hard and strong but brittle.
Ceramics are bonded together by an ionic or covalent bond.
Ceramic tiles are highly resistant to harsh chemical agents like alkalis acids household chemicals and swimming pools salts in high and low concentrations.
Some elements such as carbon or silicon may be considered ceramics.
And an ability to take a decorative finish.
Typical properties of ceramics.
Composite materials combine two or more materials.
These structures and chemical ingredients though various result in universally recognized ceramic like properties of enduring utility including the following.
High hardness high compressive strength low thermal and electrical conductivity and chemical inertness.
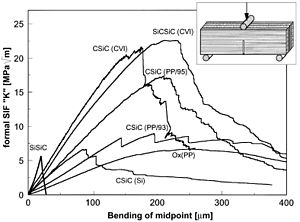
The properties of ceramics make fracturing an important inspection method.
Hardness contributing to resistance against wear.
High melting points so they re heat resistant.
Melting points high 600 4000c thermal conductivities are low insulators thermal expansion values are low 1 15 ppm c 3.
High resistance to corrosion and chemical attack.